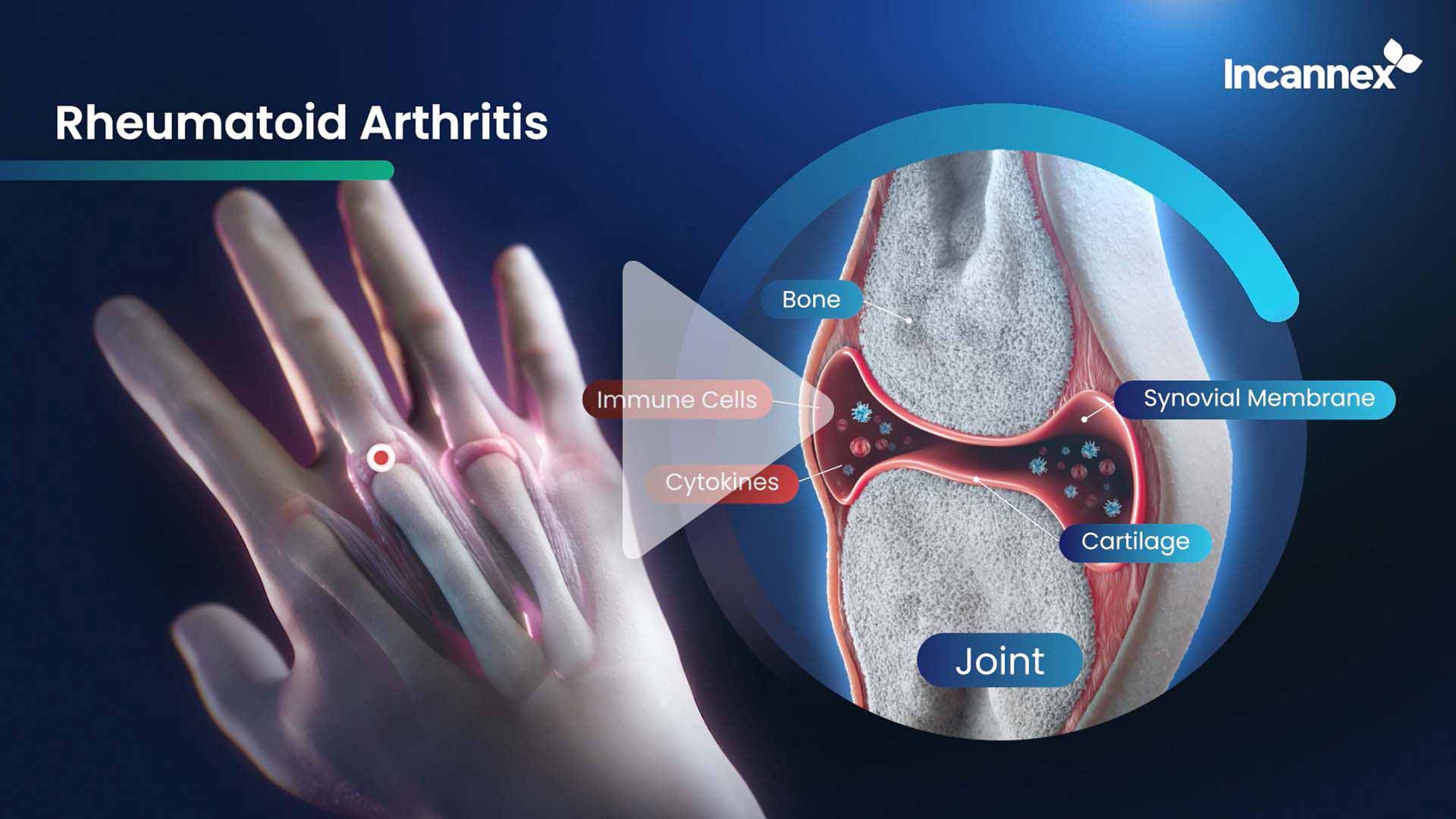
IHL-675A Rheumatoid Arthritis
About
IHL-675A is a novel cannabinoid combination product for treatment of inflammatory diseases including rheumatoid arthritis. Click below to watch IHL-675A’s progressive treatment of Rheumatoid Arthritis.
Program Overview
IHL-675A is a cannabinoid-enhanced combination drug comprising hydroxychloroquine (‘HCQ’) and cannabidiol (‘CBD’). Incannex has demonstrated that IHL-675A components, HCQ and CBD act synergistically to inhibit production of inflammatory cytokines, both in vitro and in vivo, and reduce disease severity in three established inflammatory disease animal models.
The disease models that exhibited the greatest signals of efficacy relate to rheumatoid arthritis, inflammatory lung conditions and inflammatory bowel disease. The treatment of these indications has a combined global annual market size exceeding US$125B per annum.
Incannex has completed a phase 1 clinical trial to measure the safety, tolerability, and pharmacokinetic profiles of IHL-675A compared to the FDA reference listed drugs, Plaquenil (HCQ) and Epidiolex (CBD).
Incannex has completed a phase 1 clinical trial to measure the safety, tolerability, and pharmacokinetic profiles of IHL-675A compared to the FDA reference listed drugs, Plaquenil (HCQ) and Epidiolex (CBD).
Three cohorts of 12 participants (n=36) received either IHL-675A, CBD or HCQ and the clinical assessments were identical across the three arms of the trial. At the conclusion of the trial, Incannex observed IHL-675A to be well tolerated, with no adverse events of concern or serious adverse events.
IHL-675A has been shown to outperform HCQ in an animal model of rheumatoid arthritis. IHL-675A was more effective than the rodent equivalent standard dose of HCQ at reducing arthritis across multiple assessments including clinical score, paw volume, pannus score, total histology score and serum cytokine levels. The reduction in disease assessments achieved by IHL-675A were 1.06-3.52 times that observed for HCQ alone at the standard dose. These promising observations have led Incannex to prioritise rapid clinical assessment of IHL-675A.
HCQ was first approved by FDA in 1955 and is a long-time, proven treatment option for patients with rheumatoid arthritis. Anecdotally, many patients are also reportedly using non-cGMP grade CBD to ameliorate their symptoms. To test IHL-675A’s outperformance of HCQ in animal studies, Incannex is undertaking a 120-patient phase 2 clinical trial to assess its proprietary fixed dose combination of HCQ and CBD (IHL-675A) with the goal to achieve FDA marketing approval for a pharmaceutical grade IHL-675A product that can be prescribed by a patient’s doctor.
Addressable Market
Rheumatoid arthritis drugs market is expected to reach USD $62.9 billion by 2027. (6)
6 https://www.alliedmarketresearch.com/rheumatoid-arthritis-RA-drugs-market
Development
Incannex is currently undertaking a phase 2, blinded, placebo-controlled clinical trial to determine the safety and efficacy on pain and function of IHL-675A cGMP soft gel capsules in patients with rheumatoid arthritis. The multi-site trial is recruiting 120 patients in total and is assessing patient reported outcomes, disease scores and inflammatory biomarker analysis over a 24-week period.
Patients, who meet the criteria for joint damage, may also be included in a magnetic resonance imaging (MRI) assessment sub-study. The results of the trial will establish the safety and efficacy of IHL-675A and contribute to the combination rule assessment in a FDA505(b)2 new drug application dossier.
IHL-675A comprises a combination of HCQ, an expired patent registered pharmaceutical drug, and CBD. HCQ is a disease modifying anti-rheumatic drug that regulates the activity of the immune system, which may be overactive in some conditions. HCQ can modify the underlying disease process, rather than simply treating the symptoms.
Incannex has demonstrated that IHL-675A components, CBD and HCQ act synergistically to inhibit production of key inflammatory cytokines in an in vitro study of human cells and in four distinct successful in vivo experiments using established models of inflammation.
Incannex has evaluated the results of these experiments and believe IHL-675A to be a multi-use drug candidate suitable for the prevention and treatment of inflammation, with an initial focus on: rheumatoid arthritis, inflammatory lung conditions (acute respiratory distress syndrome, COPD, asthma, and bronchitis), and inflammatory bowel disease.
In a pre-IND meeting, the US FDA has agreed that marketing applications for IHL-675A should be 505(b)(2) applications. A 505(b)(2) New Drug Application (‘NDA’) contains full safety and effectiveness reports but allows some of the information required for NDA approval, such as safety and efficacy information on the active ingredients, to originate from historical studies not conducted by Incannex.
This will result in an accelerated and less-costly route to approval, compared with a traditional development path [505(b)(1)], whilst creating new and differentiated commercial products, subject to clinical success.