IHL-42X Obstructive Sleep Apnoea (OSA)
About
IHL-42X is a novel cannabinoid combination product for treatment of Obstructive Sleep Apnoea (OSA). Click below to watch IHL-42X’s progressive treatment.
Program overview
Incannex is developing a pill-form, user-friendly treatment for obstructive sleep apnea because the current standard of care treatment, the Continuous Positive Airway Pressure machine, entails poor patient compliance due to severe discomfort.
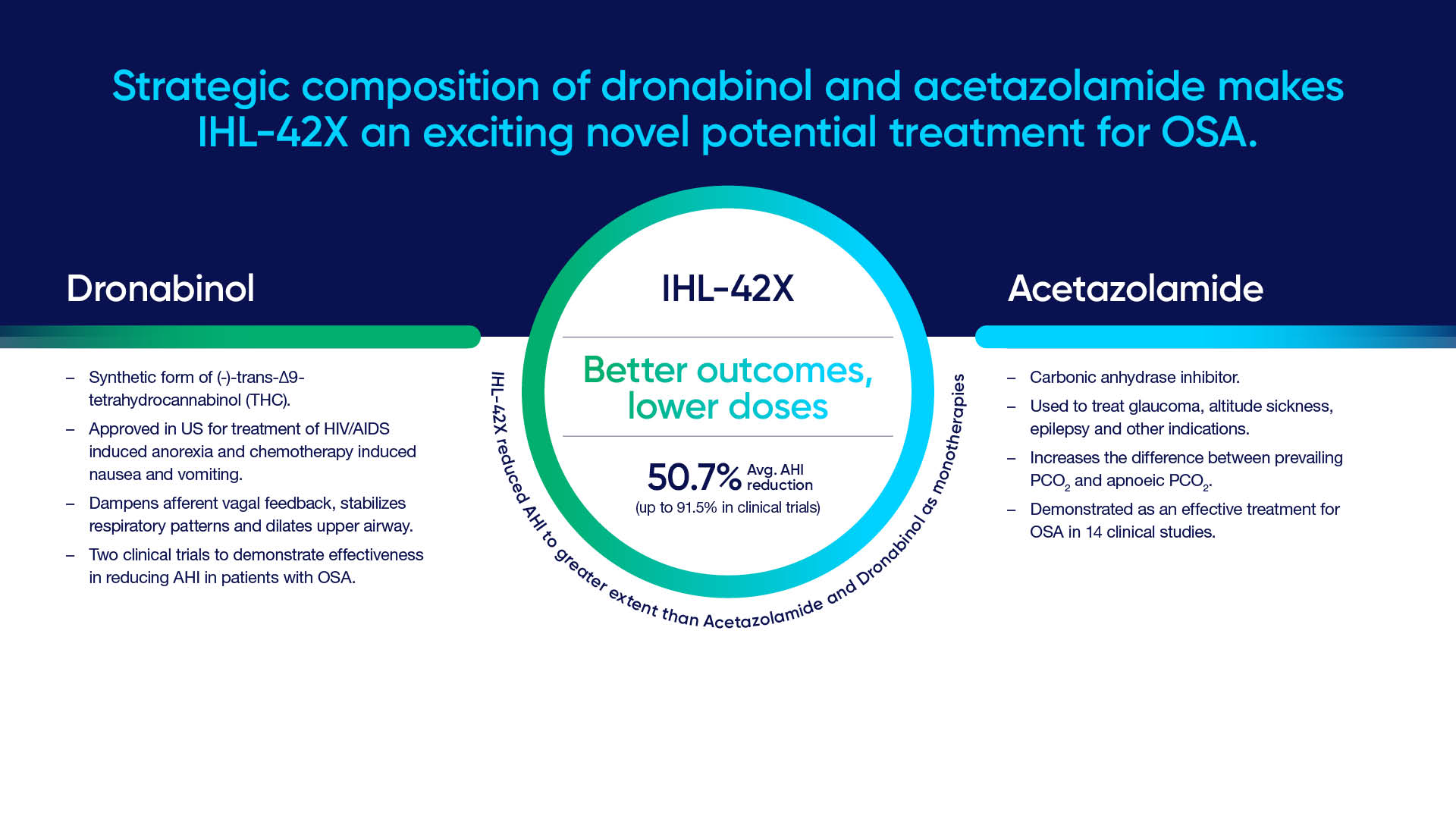
IHL-42X is a proprietary synergistic composition drug comprising low-dose dronabinol (2.5mg), a synthetic form of Tetrahydrocannabinol (THC), and acetazolamide (125mg), a carbonic anhydrase inhibitor.
IHL-42X is a proprietary synergistic composition drug comprising low-dose dronabinol (2.5mg), a synthetic form of Tetrahydrocannabinol (THC), and acetazolamide (125mg), a carbonic anhydrase inhibitor.
Results from a phase 2 proof of concept clinical trial undertaken by Incannex, with 44 data points from participants, were made public in 2022. Trial participants in the trial saw a large and statistically significant reduction in the apnea-hypopnea index (AHI) vs placebo.
Average apnea hypopnea index (AHI) was reduced from 42.8 to 21.1 events/hour in the IHL-42X arm, a 50.7% reduction in AHI. Furthermore, 60% of participants experienced a greater than 55% reduction in AHI and 25% of treated participants displayed an 80% or greater reduction.
The AHI reduction benefit occurred across the entire night unlike CPAP that are often used for only part of the night due to discomfort experienced by the user. No serious treatment emergent adverse events were reported during the clinical trial and IHL-42X improved multiple measures of sleep quality vs placebo.
IHL-42X improved sleep efficiency, defined as the proportion of time a person is asleep in bed, reduced the number of awakenings per night, and reduced the total minutes the subject was awake during the night (WASO).
Furthermore, due to encompassing only a low dose THC, the concentration of THC in blood was well below the limit for impaired driving the morning after bedtime administration of IHL-42X.
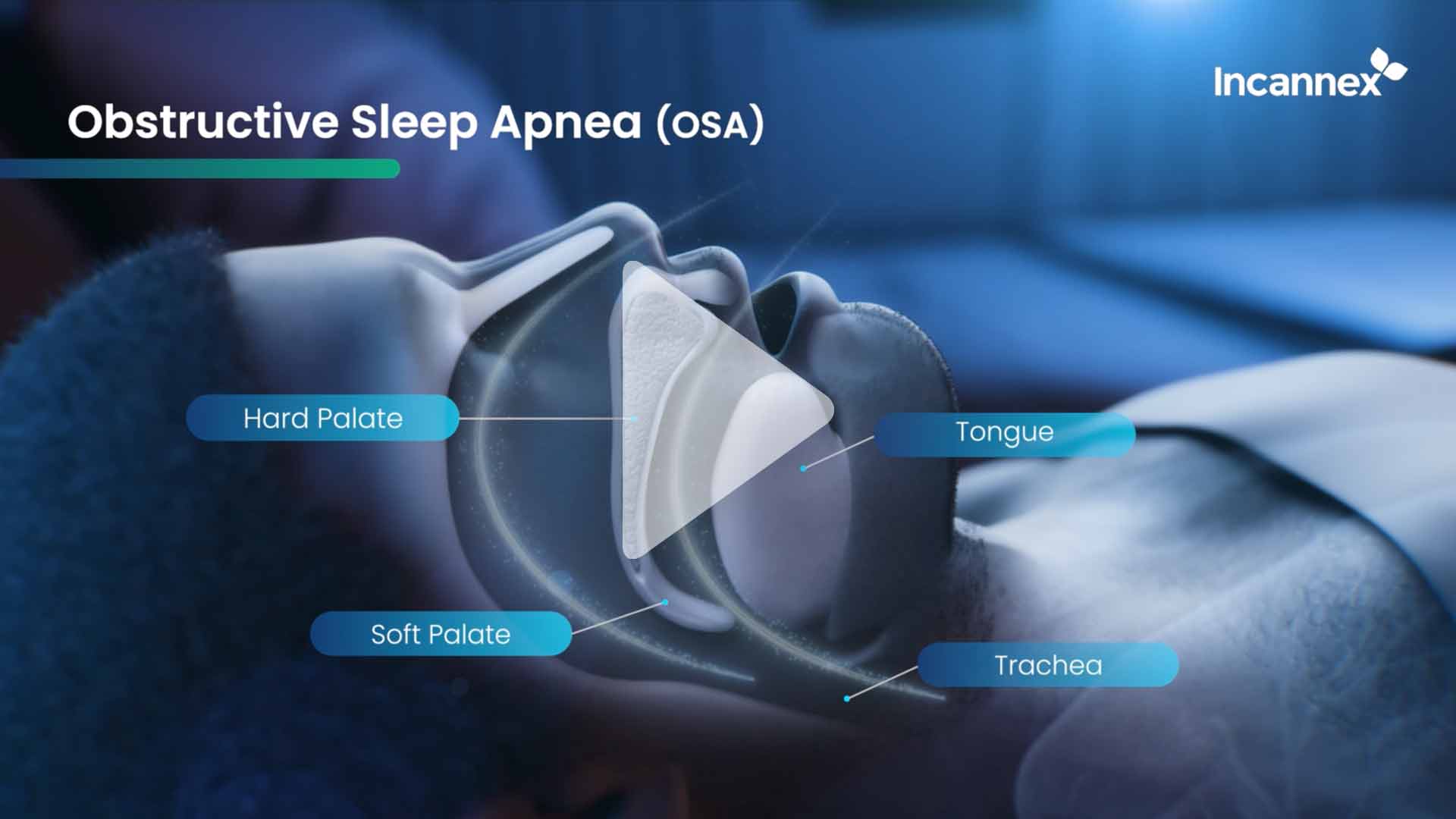
Obstructive sleep apnea is the most common sleep-related breathing disorder. It involves the narrowing of the upper airway during sleep, interfering with a person’s breathing, decreasing oxygen uptake, resulting in poor-quality sleep.
Untreated sleep apnea leads to serious long-term adverse health outcomes including hypertension, cardiovascular disease, heart attack, cognitive impairments, anxiety and depression, irritability and daytime fatigue increasing the risk of accidents. There are no pharmacotherapy (drug) treatments available to those afflicted. The current ‘standard of care’ is the Continuous Positive Airway Pressure (CPAP) machine. However, patient compliance to CPAP is low due to various factors related to patient discomfort.
Incannex anticipates greatly improved treatment compliance and outcomes from a pharmaceutical product, such as IHL-42X. Regardless of the discomfort caused by CPAP, the global annual market for OSA detection and treatment using CPAP and other breathing aids is approximately US$10 billion per annum and growing. Obstructive sleep apnea is highly prevalent, affecting approximately 30 million adults in the United States alone. It is estimated that the annual economic burden of undiagnosed sleep apnoea among U.S. adults is approximately US$149.6 billion per annum. These costs include US$86.9 billion in lost productivity, US$26.2 billion in motor vehicle accidents and US$6.5 billion in workplace accidents.
Addressable Market
- It is estimated that 936 million people have OSA globally. (1)
- Estimated sleep apnoea device market in 2021 is USD $3.9 billion. (2)
1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7007763/
2 https://www.grandviewresearch.com/industry-analysis/sleep-apnea-devices-market/
Development
As observed in a phase 2 proof of concept trial (described in the overview), IHL-42X is the most promising pharmacological solution for obstructive sleep apnea currently under development anywhere in the world.
Incannex has completed a pre-IND meeting to discuss its long-term development strategy with the US FDA and is working on producing the requisite study data for a 505(b)(2) new drug application.
Incannex has completed a pre-IND meeting to discuss its long-term development strategy with the US FDA and is working on producing the requisite study data for a 505(b)(2) new drug application.
Incannex is currently undertaking a bioavailability/bioequivalence (BA/BE) study to assess the pharmacokinetics and tolerability of IHL-42X. The study includes 116 participants who each complete four (4) single dose treatment periods, being dosed with IHL-42X, dronabinol and acetazolamide under fasted conditions as well as IHL-42X under fed conditions.
Blood samples will be collected over 48 hours and the concentrations of the APIs and their major metabolites in the samples will be analysed. The design of the BA/BE study is consistent with FDA recommendations for BA/BE and specific advice received by Incannex in the pre-IND meeting. The study will be conducted at CMAX Clinical Research in Adelaide, South Australia and managed by Novotech.
Blood samples will be collected over 48 hours and the concentrations of the APIs and their major metabolites in the samples will be analysed. The design of the BA/BE study is consistent with FDA recommendations for BA/BE and specific advice received by Incannex in the pre-IND meeting. The study will be conducted at CMAX Clinical Research in Adelaide, South Australia and managed by Novotech.
In parallel with the BA/BE study, Incannex is preparing an IND application for submission to the FDA. Once the IND is opened, it is continuously updated with research and development results for the purpose of ongoing assessment by the FDA. Incannex aims to submit the IND application in 1H 2023, followed shortly by the commencement of pivotal, multi-site, phase 2/3 clinical trials investigating the efficacy of IHL-42X in patients with obstructive sleep apnea over a 12-month period.
All participants will complete daily surveys on their sleep quality, attend monthly clinic visits to assess functional outcomes of sleep, cognitive function and other measures of safety and efficacy. Every 3 months, overnight polysomnography will be conducted to determine the effect of treatment on the patients’ AHI.
In preparation for the Phase 2/3 clinical trials, Incannex has undertaken a 12-week feasibility study whereby the trial design was provided to potential investigators along with a survey to gauge interest in conducting the study and identify any region-specific regulatory hurdles. This study involved contacting 195 sites across 14 countries in North America, Europe, South America, and Australasia. Sixty-three sites expressed interest in conducting the study. Incannex anticipates that 20-30 sites will eventually be selected to conduct the clinical trials.